eWater Electrolysis

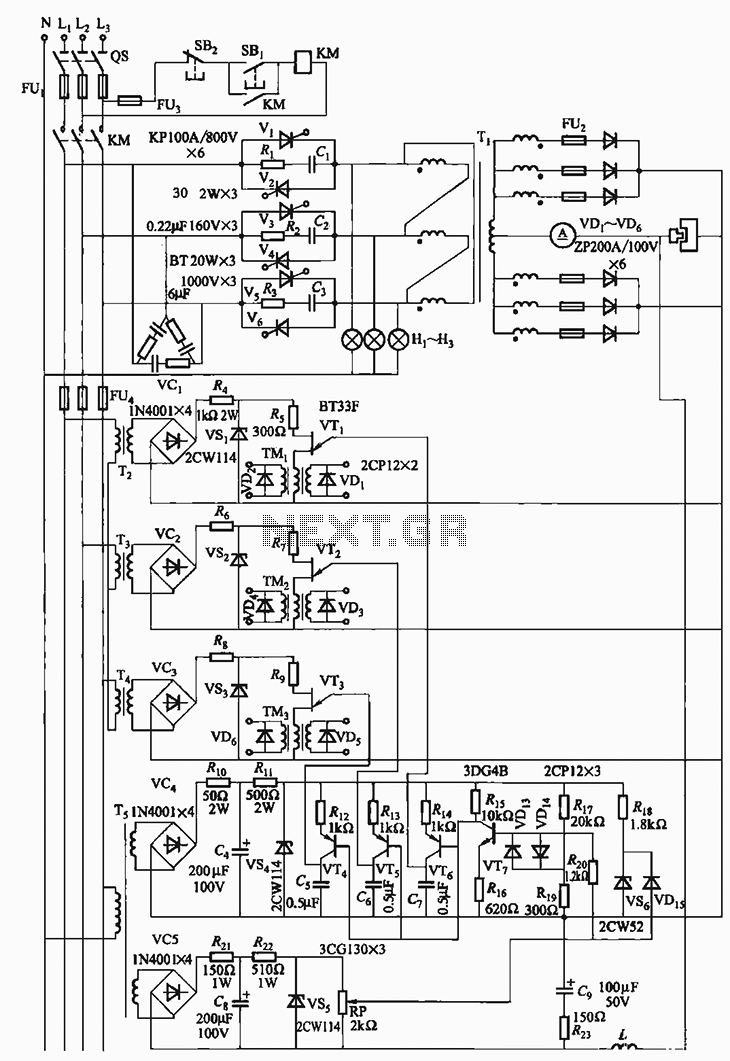
Using four batteries, similar to those found in a flashlight, it is possible to produce hydrogen and oxygen at a rate equivalent to approximately 42 liters of regular-grade fuel per hour. While the batteries remain functional, they can generate a total calorific value corresponding to at least 252 liters of regular-grade fuel. For a vehicle that consumes about 10 liters per 100 kilometers (62 miles), this setup could theoretically allow for a travel distance of around 2500 kilometers (1553 miles). The author has received inquiries suggesting that they conducted the experiment, but they clarify that they have only reproduced the report available on the website. The author references a high-frequency electrolysis link from energy21 and mentions being a member of an inventor's cooperative in Northern California, with a keen interest in researching more efficient electrolysis methods for on-demand hydrogen production for vehicle power. They have accessed Puharich's patents, a white paper, the Xogen patent, and other relevant information. Attempting to replicate the reported results from the high-frequency electrolysis page yielded different outcomes. The experiment utilized an existing power circuit designed by an electronic engineer. A solution of 10% battery acid was prepared by adding 30ml of acid to 270ml of tap water in a plastic beaker, using two 24k gold-plated copper electrode coils connected to a power source. The power source was current-limited to 250mA, with a supply voltage of 12V; however, the voltage across the cell fluctuated between 1 to 2 volts due to changing cell impedance. As the frequency of the power source varied from 6Hz to over 1300Hz, no change in gas production was observed, although bubble size varied significantly. The author speculates that the original circuit may have been voltage-regulated, allowing current to vary with the cell's impedance at different frequencies, potentially explaining the discrepancies in gas production. The author expresses a strong interest in finding more efficient electrolysis methods for producing inexpensive hydrogen and seeks collaboration to address the differences in experimental results. They also note skepticism regarding the validity of results presented on the referenced page, citing an email received that raises concerns.
In the context of electrolysis for hydrogen production, the described experiment presents a setup involving four batteries, which serve as the energy source for the electrolysis process. The electrolysis cell is constructed using gold-plated copper electrodes to enhance conductivity and minimize corrosion, which is critical for long-term operation. The choice of a 10% sulfuric acid solution as the electrolyte is significant, as sulfuric acid is known to increase the conductivity of water, facilitating the electrolysis process.
The power supply is designed to limit current to 250mA, which is essential for controlling the electrolysis rate and preventing excessive gas production that could lead to safety hazards. The supply voltage of 12V, while nominal, varies due to the changing impedance of the electrolysis cell, which is influenced by factors such as electrode surface area, electrolyte concentration, and temperature. The frequency variation from 6Hz to 1300Hz suggests an exploration of high-frequency electrolysis, which is theorized to enhance gas production through improved ion movement and bubble dynamics.
The observation that bubble size changes without a corresponding increase in gas production indicates that while frequency affects bubble behavior, it may not directly correlate with the efficiency of hydrogen and oxygen generation. This distinction is critical for optimizing the electrolysis process, as it suggests that other factors, such as electrode design and electrolyte composition, may play more significant roles in enhancing gas output.
The inquiry into whether the original circuit was voltage-regulated reflects a deeper understanding of electrolysis dynamics. If the voltage across the cell is maintained while allowing the current to vary, the system could potentially adapt to changes in cell impedance more effectively, leading to improved gas production rates. Collaborative efforts to share findings and methodologies could lead to advancements in efficient hydrogen production techniques, which are vital for developing sustainable energy solutions for transportation and other applications.With 4 batteries (as for instance in the flashlight) it is possible to produce per hour hydrogen and oxygen in a quantity which corresponds to regular grade fuel in approximately 42 litres. Until the batteries are exhausted, they produced for calorific value, which corresponds at least 252 litres to regular grade fuel.
Converted a vehicle, which uses into approximately 10 litres on 100 km (62 miles), with 4 commercial batteries could drive about 2500 (1553 miles) kilometers far. I had some emails assuming that the web owner (me) has performed the above experiment, I have not done so and have merely reproduced the report at this site.
I reference to the high frequency electrolysis link on the energy21. I`m a member of an inventor`s coop in Northern California and am very interested in researching more efficient means of electrolysis for producing hydrogen on demand for powering vehicles. I have accessed Puharich`s patents and white paper and the Xogen patent and other information which you might also find interesting.
I thought of starting with something simple like trying to obtain similar results to your experiment. In trying to replicate or at least approximate the results found in your page on high frequency electrolysis we had very different results.
We utilized an existing power circuit designed by a friend, an electronic engineer. We started with a solution of 10% battery acid, commercially available in the US, It is not clear to us what concentration of sulfuric acid this is, but we added 30ml of this to 270ml of tap water in a plastic beaker. We then used two 24k gold plated copper electrode coils attatched to our power source. The power source was CURRENT LIMITED to 250mA, the voltage supplied to the circuit was 12V, but as measured at the cell it varied from 1 to 2 volts as the impedance of the cell changed.
The cell impedance changed when we varied the frequency of the power source from. 6Hz to over 1300HZ. In doing so, we found no change whatsoever in the amount of gas produced as we varied frequency. The only difference we found was that the bubble size changed from large (approx. 5cm) to very small. Because we found that the voltage across the cell varied with frequency we are wondering if perhaps your circuit was voltage requlated with the current varying freely in response to the impedance load of the cell as the frequency varied If so, this could explain the dramatic difference in gas produced due to current variance. I am very interested in more efficient electrolysis of water to produce cheap hydrogen and am wondering what we might share to resolve the differences in experimental results Please note: WE know are led to believe the above results presented on this page are a fraud the following email I that I received below seems to give the reason why
🔗 External reference
In the context of electrolysis for hydrogen production, the described experiment presents a setup involving four batteries, which serve as the energy source for the electrolysis process. The electrolysis cell is constructed using gold-plated copper electrodes to enhance conductivity and minimize corrosion, which is critical for long-term operation. The choice of a 10% sulfuric acid solution as the electrolyte is significant, as sulfuric acid is known to increase the conductivity of water, facilitating the electrolysis process.
The power supply is designed to limit current to 250mA, which is essential for controlling the electrolysis rate and preventing excessive gas production that could lead to safety hazards. The supply voltage of 12V, while nominal, varies due to the changing impedance of the electrolysis cell, which is influenced by factors such as electrode surface area, electrolyte concentration, and temperature. The frequency variation from 6Hz to 1300Hz suggests an exploration of high-frequency electrolysis, which is theorized to enhance gas production through improved ion movement and bubble dynamics.
The observation that bubble size changes without a corresponding increase in gas production indicates that while frequency affects bubble behavior, it may not directly correlate with the efficiency of hydrogen and oxygen generation. This distinction is critical for optimizing the electrolysis process, as it suggests that other factors, such as electrode design and electrolyte composition, may play more significant roles in enhancing gas output.
The inquiry into whether the original circuit was voltage-regulated reflects a deeper understanding of electrolysis dynamics. If the voltage across the cell is maintained while allowing the current to vary, the system could potentially adapt to changes in cell impedance more effectively, leading to improved gas production rates. Collaborative efforts to share findings and methodologies could lead to advancements in efficient hydrogen production techniques, which are vital for developing sustainable energy solutions for transportation and other applications.With 4 batteries (as for instance in the flashlight) it is possible to produce per hour hydrogen and oxygen in a quantity which corresponds to regular grade fuel in approximately 42 litres. Until the batteries are exhausted, they produced for calorific value, which corresponds at least 252 litres to regular grade fuel.
Converted a vehicle, which uses into approximately 10 litres on 100 km (62 miles), with 4 commercial batteries could drive about 2500 (1553 miles) kilometers far. I had some emails assuming that the web owner (me) has performed the above experiment, I have not done so and have merely reproduced the report at this site.
I reference to the high frequency electrolysis link on the energy21. I`m a member of an inventor`s coop in Northern California and am very interested in researching more efficient means of electrolysis for producing hydrogen on demand for powering vehicles. I have accessed Puharich`s patents and white paper and the Xogen patent and other information which you might also find interesting.
I thought of starting with something simple like trying to obtain similar results to your experiment. In trying to replicate or at least approximate the results found in your page on high frequency electrolysis we had very different results.
We utilized an existing power circuit designed by a friend, an electronic engineer. We started with a solution of 10% battery acid, commercially available in the US, It is not clear to us what concentration of sulfuric acid this is, but we added 30ml of this to 270ml of tap water in a plastic beaker. We then used two 24k gold plated copper electrode coils attatched to our power source. The power source was CURRENT LIMITED to 250mA, the voltage supplied to the circuit was 12V, but as measured at the cell it varied from 1 to 2 volts as the impedance of the cell changed.
The cell impedance changed when we varied the frequency of the power source from. 6Hz to over 1300HZ. In doing so, we found no change whatsoever in the amount of gas produced as we varied frequency. The only difference we found was that the bubble size changed from large (approx. 5cm) to very small. Because we found that the voltage across the cell varied with frequency we are wondering if perhaps your circuit was voltage requlated with the current varying freely in response to the impedance load of the cell as the frequency varied If so, this could explain the dramatic difference in gas produced due to current variance. I am very interested in more efficient electrolysis of water to produce cheap hydrogen and am wondering what we might share to resolve the differences in experimental results Please note: WE know are led to believe the above results presented on this page are a fraud the following email I that I received below seems to give the reason why
🔗 External reference