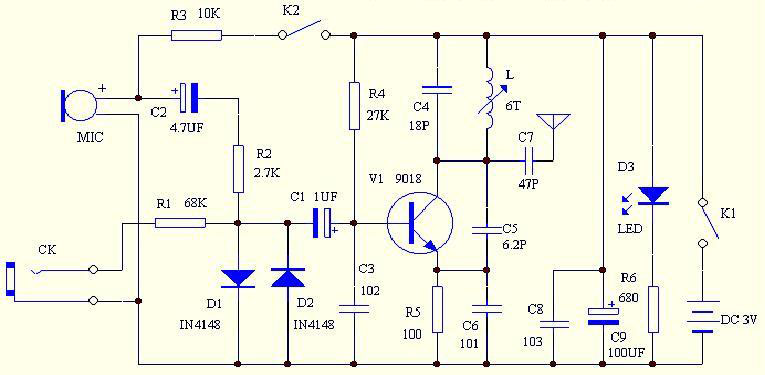
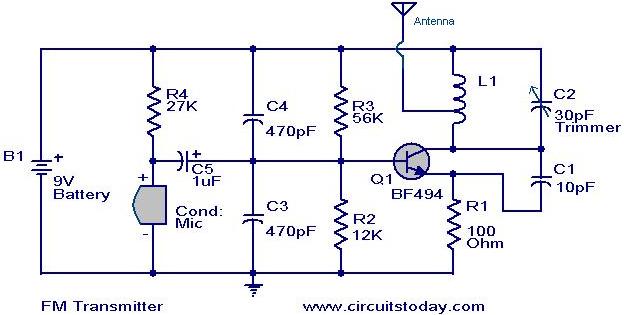
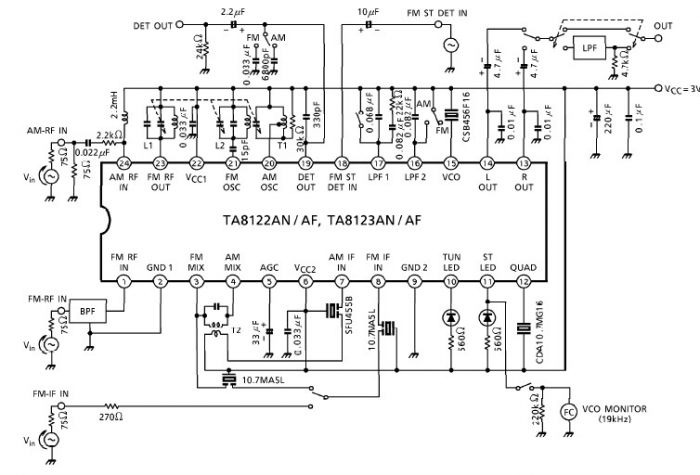
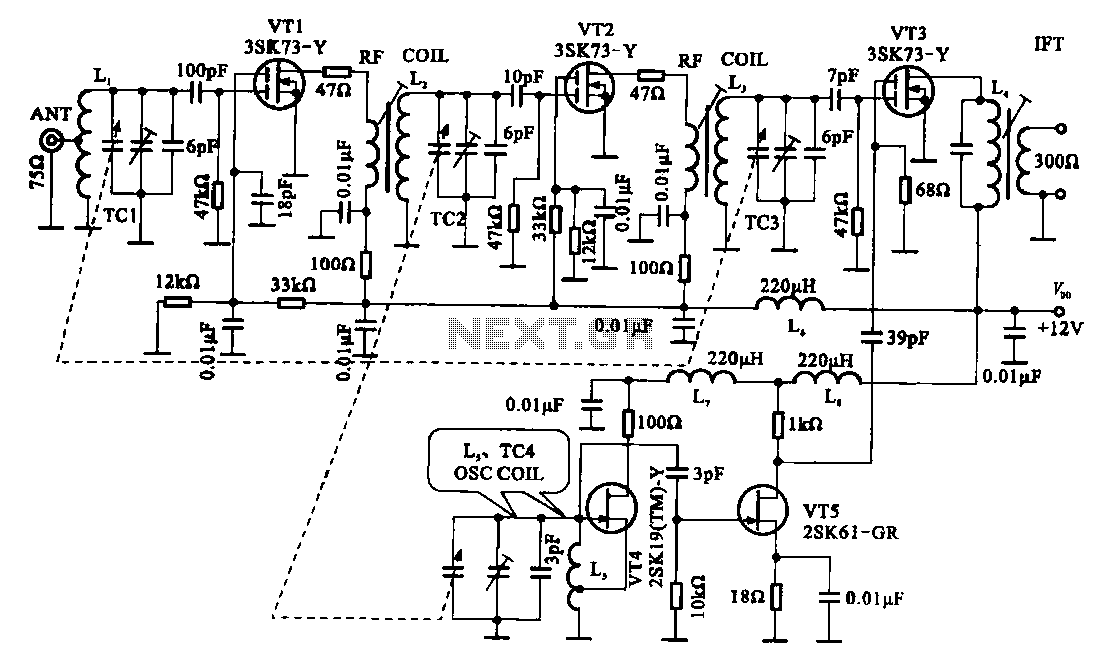
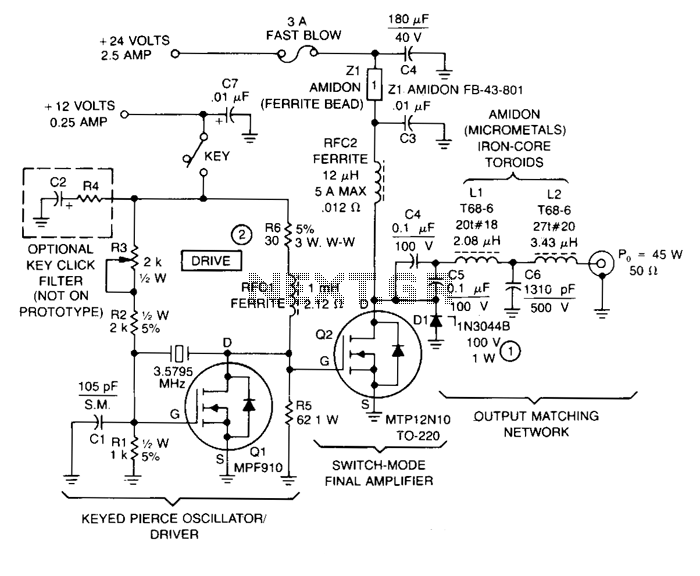
Energy-sucking Radio Antennas
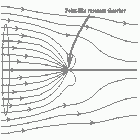
Here's something that has always bugged me: light waves are about 5000 Angstroms in wavelength, while atoms are more like 1 Angstrom across. Atoms are thousands of times smaller than light waves, yet atoms obviously interact very strongly with light. How can they do this? Perhaps they get around the problem by employing Quantum Mechanics (photon-physics rather than EM waves?) There must be some explanation. More: After all, when a metal dipole antenna is only one foot long, it certainly cannot absorb much 5000ft-wave radiation. I never encountered a good explanation for this during my physics education. I finally found a couple of physics papers that make things c
The interaction between light waves and atoms is a fundamental concept in quantum mechanics and electromagnetism. Light waves, or electromagnetic waves, have a wavelength in the range of thousands of Angstroms, while the size of an atom is typically around 1 Angstrom. This significant size disparity raises questions about how atoms can effectively interact with light.
The explanation lies in the quantum nature of light and matter. Light can be described not only as a wave but also as a stream of particles called photons. When light interacts with atoms, it does so at specific energy levels that correspond to the electronic transitions within the atom. When a photon of light encounters an atom, it can be absorbed if its energy matches the energy difference between two electron energy levels in the atom. This is a key principle of quantum mechanics.
In practical applications, such as in antennas, the size and design of the antenna must correspond to the wavelength of the electromagnetic radiation it intends to receive or transmit. A metal dipole antenna, for instance, typically operates at wavelengths that are several times longer than its physical length. The antenna's design allows it to resonate at specific frequencies, enabling it to interact with electromagnetic waves effectively, even if its physical size is smaller than the wavelength of the radiation.
This phenomenon can also be observed in various optical devices, such as lasers and photodetectors, where the interaction between light and matter is harnessed for specific applications. Understanding these interactions is crucial for advancements in fields such as telecommunications, spectroscopy, and quantum computing. The principles governing these interactions are foundational to the development of modern electronic and photonic devices, illustrating the intricate relationship between light and atomic structures.Here's something that has always bugged me: light waves are about 5000 Angstroms in wavelength, while atoms are more like 1 Angstrom across. Atoms are thousands of times smaller than light waves, yet atoms obviously interact very strongly with light.
How can they do this? Perhaps they get around the problem by employing Quantum Mechanics (photon-physics rather than EM waves?) There must be some explanation. After all, when a metal dipole antenna is only one foot long, it certainly cannot absorb much 5000ft-wave radiation. I never encountered a good explanation for this during my physics education. I finally found a couple of physics papers that make things c 🔗 External reference
The interaction between light waves and atoms is a fundamental concept in quantum mechanics and electromagnetism. Light waves, or electromagnetic waves, have a wavelength in the range of thousands of Angstroms, while the size of an atom is typically around 1 Angstrom. This significant size disparity raises questions about how atoms can effectively interact with light.
The explanation lies in the quantum nature of light and matter. Light can be described not only as a wave but also as a stream of particles called photons. When light interacts with atoms, it does so at specific energy levels that correspond to the electronic transitions within the atom. When a photon of light encounters an atom, it can be absorbed if its energy matches the energy difference between two electron energy levels in the atom. This is a key principle of quantum mechanics.
In practical applications, such as in antennas, the size and design of the antenna must correspond to the wavelength of the electromagnetic radiation it intends to receive or transmit. A metal dipole antenna, for instance, typically operates at wavelengths that are several times longer than its physical length. The antenna's design allows it to resonate at specific frequencies, enabling it to interact with electromagnetic waves effectively, even if its physical size is smaller than the wavelength of the radiation.
This phenomenon can also be observed in various optical devices, such as lasers and photodetectors, where the interaction between light and matter is harnessed for specific applications. Understanding these interactions is crucial for advancements in fields such as telecommunications, spectroscopy, and quantum computing. The principles governing these interactions are foundational to the development of modern electronic and photonic devices, illustrating the intricate relationship between light and atomic structures.Here's something that has always bugged me: light waves are about 5000 Angstroms in wavelength, while atoms are more like 1 Angstrom across. Atoms are thousands of times smaller than light waves, yet atoms obviously interact very strongly with light.
How can they do this? Perhaps they get around the problem by employing Quantum Mechanics (photon-physics rather than EM waves?) There must be some explanation. After all, when a metal dipole antenna is only one foot long, it certainly cannot absorb much 5000ft-wave radiation. I never encountered a good explanation for this during my physics education. I finally found a couple of physics papers that make things c 🔗 External reference