NiCd/NiMH Battery Charger
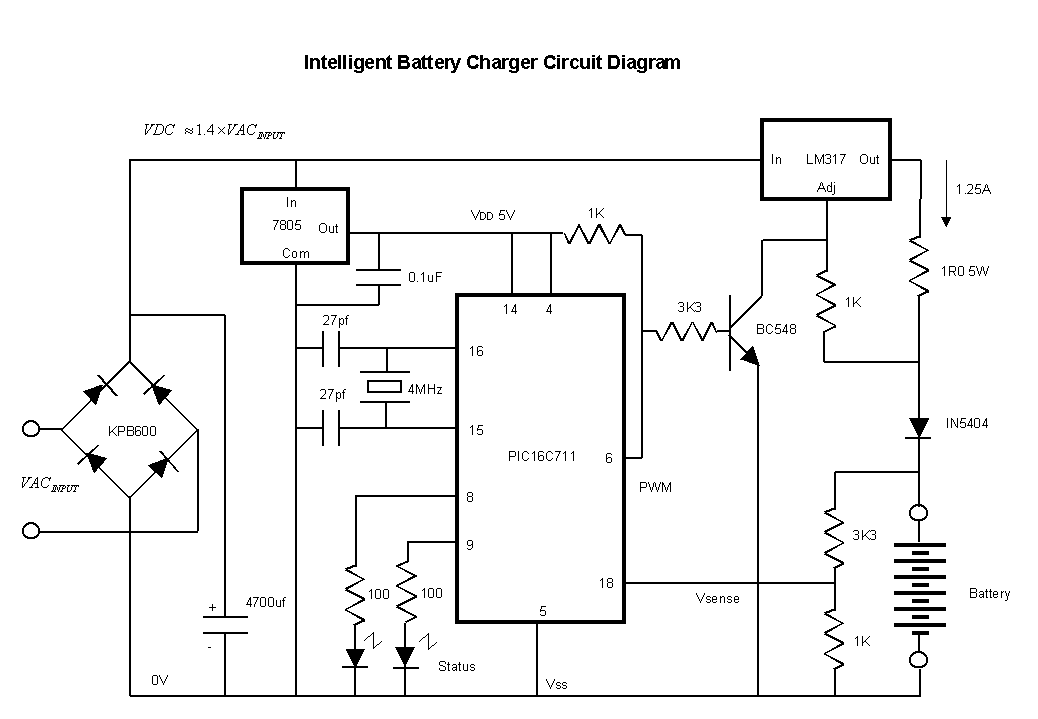
Here we use the PIC16711. Rechargeable battery capacity is rated in mAH (milliampere-hours). The total capacity of a battery is defined as "C", that is it can supply C mA for 1 hour, or 2C for 30 minutes etc. Charge rates can vary from trickle charges to keep the battery 'topped up' of 3.3% of C to 5% of C, a slow current charge of 10% of C to 20% of C or a fast charge of 50% of C to 100% of C. Slow charges are not meant to be continually applied, and since NiCd/NiMH batteries are about 66% efficient, they normally last about 8-15 hours. Fast charges such as 100% of C should be terminated after about 1.5 hours, providing the battery is flat to begin with. Once a battery is fully charged, the battery produces gas creating a high internal pressure, and a sudden rise in temperature. The charge should be switched to a trickle charge at this point or the battery will begin to vent and release its electrolyte. My old battery was rated at C=1300mAH and my old charger was rated 400mA (30% of C) so the charger should have been switched off after about 4 hours, provided that they were almost flat to begin with. However there is no way of knowing if C was actually 1300maH or if it had decreased a bit, and once the a battery starts to deteriorate, I suspect this becomes a vicious cycle and the battery deteriorates rapidly due to more and more overcharging. The manufacturer suggests these cells should be good for 500 to 1000 cycles if properly treated!
The Memory Effect Myth
Possibly the biggest myth that exists particularly for NiCd cells is the "memory effect". Almost everyone quotes it as the reason that cells have to be completely flattened - otherwise they develop some sort of memory and can only hold a partial charge from there on. Like all good stories, this one has a grain of truth in it! The myth originated from the early days of satellites when they were using solar cells to charge batteries and because of the orbiting of the craft around the earth, the batteries were subjected to precise charge/discharge cycles many hundreds of times. The effect disappears when the battery cycle is suddenly varied, and it is extremely difficult to reproduce this effect even in a laboratory. So the "memory effect" is not a significant problem in home usage. What can be noted is while it may be acceptable to discharge individual cells to 0V, it is certainly not recommended to discharge an entire battery of cells. The reason is simple. When the battery is discharged below 0.8V per cell, one of the cells is inevitably weaker than the others, and goes to zero first. If the battery is further flattened, this battery becomes charged in reverse, which again makes it still weaker. This creates a more common but less commonly known effect called "voltage depression". Eventually the battery's performance drops off quite suddenly which ironically is the very thing that the user is trying to prevent. Most users know where the battery's "knee" occurs; it is when the original equipment first starts to show signs that the battery performance (and hence voltage) is suddenly dropping, and it is a good idea to place it straight on charge at this point. Usually there is less than 5% of C remaining anyway.
One other thing, batteries don't like getting too hot or cold; they do not take a full charge and they actually discharge (even under no load) much faster when over 40 degrees or below 0 degrees. They can build up internal heat when working and this can cause temperatures inside to increase also. Particularly avoid leaving cordless tools inside a hot car for this reason. They also should be left to cool down for a while after discharge before placing them on charge. NiCd/NiMH batteries do self-discharge too, as a rule of thumb a battery will hold a full charge (with no load) for about a month or two, although when they get old or hot, they might only last a day.
So what can be learned from this?
You don't have to flatten your battery before you recharge it,
Don't flatten your battery below 0.8V per cell,
Don't overcharge your battery beyond 100% of C, and
NiCd/NiMH don't like to get too hot, or too cold (0 to 40 degrees C is usually best)
NiCd/NiMH Charging
Common values for C for cordless tools and racing cars are in the range from 1000maH to 3000maH. The first step is to determine what C is for your cells. Inspect the cells or contact the manufacturer to determine the cell part number. In drills, the battery packs can often be disassembled easily. The value for C often forms some of the part number and the part number can be searched for on the Internet. For a new battery, the value for C was 1700maH. Note that the cell value for C is the same as the battery value for C. Manufacturers' data gives that when designing a charger, it is essential to first consider how the cells are to be used. For these applications, the charge use is termed "cycle use", where the battery is repeatedly charged and discharged. In addition, usually the charge time required is as fast as possible, between 1 and 2 hours. Batteries may be capable of taking a fast charge of 100% of C, which equates to 1.7A. Despite this, it is often advisable to select a charge current that is slightly lower than 100% of C to accommodate various battery capacities.
For "cycle use", two recommended methods of detecting charge termination are available: using a temperature sensor in the battery pack or employing a "negative delta V" cutoff system. The temperature technique relies on detecting a sudden rise in battery temperature to shut off the charge. There is nothing inherently wrong with this approach, but battery packs do not always come with temperature sensors built in. Furthermore, those that do often sense the temperature of only one cell.
The negative delta V system utilizes the electrical characteristic that the NiCd/NiMH battery voltage peaks and drops about 20mV per cell when fully charged. This charger, in its basic configuration, will detect a peak of 84mV (per battery) from 2V to 21.5V, thus capable of charging any battery pack within this range (i.e., 6-12 cells or 7.2V to 14.4V). Consequently, no matter how discharged the battery is, this technique will provide sufficient charge to restore the battery to its full state, followed by a continuous "topping up" with a trickle charge to mitigate slow leakage through internal resistance.
Additional considerations include the necessity of allowing a battery to cool down before applying a charge for optimal performance. The charger is designed to wait for the battery voltage to stabilize for approximately 30 seconds before initiating charging. If the battery has just come off discharge and is hot, it may take a minute or so for the charge to commence. Additionally, new batteries may exhibit false peaks during the initial 4 minutes of charging; therefore, the charger begins with a slow "soft start" charge for 4 minutes to allow the battery to cool and surpass this transient period.
The charger employs a threshold of 2V (open circuit voltage) to recognize the connection of a battery. In practice, even a very old battery that has been shorted out for some time will recover above this value when unloaded. The charging algorithm utilized by the PIC is structured to ensure systematic operation. During normal operation, the charger is activated, and both LEDs will flash once. The charger will then enter mode 0 (standby) until a battery is connected. Upon connection, the charger progresses through mode 1 (cool), mode 2 (soft), mode 3 (fast), and mode 4 (trickle). The battery can remain in mode 4 (trickle) indefinitely or be removed at that point. When the battery is removed, the charger will revert to mode 0 (standby).Here we use the PIC16711. Rechargeable battery capacity is rated in mAH (milliampere-hours). The total capacity of a battery is defined as "C", that is it can supply C mA for 1 hour, or 2C for 30 minutes etc. Charge rates can vary from trickle charges to keep the battery 'topped up' of 3.3% of C to 5% of C, a slow current charge of 10% of C to 20% of C or a fast charge of 50% of C to 100% of C.
Slow charges are not meant to be continually applied, and since NiCd/NiMH batteries are about 66% efficient, they normally last about 8-15 hours. Fast charges such as 100% of C should be terminated after about 1.5 hours, providing the battery is flat to begin with.
Once a battery is fully charged, the battery produces gas creating a high internal pressure, and a sudden rise in temperature. The charge should be switched to a trickle charge at this point or the battery will begin to vent and release its electrolyte. My old battery was rated at C=1300mAH and my old charger was rated 400mA (30% of C) so the charger should have been switched off after about 4 hours, provided that they were almost flat to begin with.
However there is no way of knowing if C was actually 1300maH or if it had decreased a bit, and once the a battery starts to deteriorate, I suspect this becomes a vicious cycle and the battery deteriorates rapidly due to more and more overcharging. The manufacturer suggests these cells should be good for 500 to 1000 cycles if properly treated! The Memory Effect Myth Possibly the biggest myth that exists partcularly for NiCd cells is the "memory effect".
Almost every one quotes it as the reason that cells have to be completely flattened - otherwise they develop some sort of memory, and can only hold a partial charge from there on. Like all good stories, this one has a grain of truth in it! The myth originated from the early days of satellites when they were using solar cells to charge batteries and because of the orbiting of the craft around the earth, the batteries were subjected to precise charge/discharge cycles many hundreds of times.
The effect disappears when the battery cycle is suddenly varied, and it is extremely difficult to reproduce this effect even in a laboratory. So the "memory effect" is not a significant problem in home usage. What I can tell you is while it may be OK to discharge individual cells to 0V, it is certainly not recommended to discharge an entire battery of cells.
The reason is simple. When the battery is discharged below 0.8V per cell, one of the cells is inevitably weaker than the others, and goes to zero first. If the battery is further flattened this battery becomes charged in reverse, which again makes it still weaker.
This creates a more common but less commonly known effect called "voltage depression". Eventually the battery's performance drops off quite suddenly which ironically is the very thing that the user is trying to prevent. Most users know where the battery's "knee" occurs; it is when the original equipment first starts to show signs that the battery performance (and hence voltage) is suddenly dropping, and it is a good idea to place it straight on charge at this point.
Usually there is less than 5% of C remaining anyway. One other thing, batteries don't like getting too hot or cold; they do not take a full charge and they actually discharge (even under no load) much faster when over 40 degrees or below 0 degrees. They can build up internal heat when working and this can cause temperatures inside to increase also.
Particularly avoid leaving cordless tools inside a hot car for this reason. They also should be left to cool down for a while after discharge before placing them on charge. NiCd/NiMH batteries do self-discharge too, as a rule of thumb a battery will hold a full charge (with no load) for about a month or two, although when they get old or hot, they might only last a day. So what can you learn from this? You don't have to flatten your battery before you recharge it, Don't flatten your battery below 0.8V per cell, Don't overcharge your battery beyond 100% of C, and NiCd/NiMH don't like to get too hot, or too cold (0 to 40 degrees C is usually best) NiCd/NiMH Charging Common values for C for cordless tools and racing cars are in the range from 1000maH to 3000maH.
The first step is to determine what C is for your cells. Inspect the cells or contact the manufacturer to determine the cell part number. In drills, the battery packs can often be disassembled easily. The value for C often forms some of the part number and the part number can be searched for on the Internet. For my new battery the value for C was 1700maH. Note that the cell value for C is the same as the battery value for C. Manufacturers data gives that when designing a charger you should first consider how the cells are to be used.
For these applications the charge use is termed "cycle use" where the battery is repeatedly charged and discharged. In addition, usually the charge time required is as fast as possible, between 1 and 2 hours. My batteries were capable of taking a fast charge of 100% of C, which equates to 1.7A. Despite this I conservatively selected 1.25A as my charge current, because I wished to be able to charge 1300maH batteries also.
This value should be good for most readers, and it doesn't really matter if it is a bit less than 100% of C, because the charger will still detect a peak eventually anyway. However, some readers will want to adjust the maximum current, and this is described a bit later on. For "cycle use", there are two recommended methods of detecting charge termination, either using a temperature sensor in the battery pack or using a "negative delta V" cutoff system.
The temperature technique relies on detecting the sudden rise in battery temperature to shut off the charge. There is nothing wrong with doing this but battery packs do not always come with temperature sensors built in.
Furthermore ones that do, often sense the temperature of only one cell. The negative delta V system relies on the electrical characteristic that the NiCd/NiMH battery voltage peaks and drops about 20mV per cell when fully charged. This charger in its basic configuration will detect a peak of 84mV (per battery) from 2V to 21.5V, and thus will charge any battery pack in this range (i.e.
6-12 cells or 7.2V to 14.4V). Options to alter this range are described in Figure 10. Hence, no matter how discharged the battery is, this technique will give enough charge to restore the battery to its full state, and then the battery is continually "topped up" with a trickle charge to prevent slow leakage through internal resistance. Other things to consider are the requirement to let a battery cool down, so a better charge can be applied.
This battery charger waits for the battery voltage to stabilize for about 30 seconds before starting to charge. If the battery has just come off discharge and is hot, it may take a minute or so for the charge to begin to start.
Additionally new batteries may show false peaks in the first 4 minutes of charge. For this reason the charger starts with a slow "soft start" charge for 4 minutes to allow the battery to cool and get past this point. The charger uses a threshold of 2V (open circuit voltage) to recognize that a battery has been connected.
In practice even a very old battery that has been actually shorted out for some time, will recover above this value when unloaded. The charging algorithm used by the PIC is shown in Fig 1. Note that the first Light Emitting Diode (LED) comes on continually whilst battery is undergoing the bulk charge process, while the second LED gives an indication of the particular type of charge being applied.
Normal operation of the charger is fairly straightforward. Normally the Charger is switched on and both LEDs will flash once. The charger will then wait in mode 0 (standby) until a battery is connected. Once a battery is connected, the charger will progress though mode 1 (cool), 2 (soft), 3 (fast) and 4 (trickle). The battery can be left in mode 4 (trickle) indefinitely, or removed at this point. When the battery is removed, the charger will revert to mode 0 (standby). 🔗 External reference
The Memory Effect Myth
Possibly the biggest myth that exists particularly for NiCd cells is the "memory effect". Almost everyone quotes it as the reason that cells have to be completely flattened - otherwise they develop some sort of memory and can only hold a partial charge from there on. Like all good stories, this one has a grain of truth in it! The myth originated from the early days of satellites when they were using solar cells to charge batteries and because of the orbiting of the craft around the earth, the batteries were subjected to precise charge/discharge cycles many hundreds of times. The effect disappears when the battery cycle is suddenly varied, and it is extremely difficult to reproduce this effect even in a laboratory. So the "memory effect" is not a significant problem in home usage. What can be noted is while it may be acceptable to discharge individual cells to 0V, it is certainly not recommended to discharge an entire battery of cells. The reason is simple. When the battery is discharged below 0.8V per cell, one of the cells is inevitably weaker than the others, and goes to zero first. If the battery is further flattened, this battery becomes charged in reverse, which again makes it still weaker. This creates a more common but less commonly known effect called "voltage depression". Eventually the battery's performance drops off quite suddenly which ironically is the very thing that the user is trying to prevent. Most users know where the battery's "knee" occurs; it is when the original equipment first starts to show signs that the battery performance (and hence voltage) is suddenly dropping, and it is a good idea to place it straight on charge at this point. Usually there is less than 5% of C remaining anyway.
One other thing, batteries don't like getting too hot or cold; they do not take a full charge and they actually discharge (even under no load) much faster when over 40 degrees or below 0 degrees. They can build up internal heat when working and this can cause temperatures inside to increase also. Particularly avoid leaving cordless tools inside a hot car for this reason. They also should be left to cool down for a while after discharge before placing them on charge. NiCd/NiMH batteries do self-discharge too, as a rule of thumb a battery will hold a full charge (with no load) for about a month or two, although when they get old or hot, they might only last a day.
So what can be learned from this?
You don't have to flatten your battery before you recharge it,
Don't flatten your battery below 0.8V per cell,
Don't overcharge your battery beyond 100% of C, and
NiCd/NiMH don't like to get too hot, or too cold (0 to 40 degrees C is usually best)
NiCd/NiMH Charging
Common values for C for cordless tools and racing cars are in the range from 1000maH to 3000maH. The first step is to determine what C is for your cells. Inspect the cells or contact the manufacturer to determine the cell part number. In drills, the battery packs can often be disassembled easily. The value for C often forms some of the part number and the part number can be searched for on the Internet. For a new battery, the value for C was 1700maH. Note that the cell value for C is the same as the battery value for C. Manufacturers' data gives that when designing a charger, it is essential to first consider how the cells are to be used. For these applications, the charge use is termed "cycle use", where the battery is repeatedly charged and discharged. In addition, usually the charge time required is as fast as possible, between 1 and 2 hours. Batteries may be capable of taking a fast charge of 100% of C, which equates to 1.7A. Despite this, it is often advisable to select a charge current that is slightly lower than 100% of C to accommodate various battery capacities.
For "cycle use", two recommended methods of detecting charge termination are available: using a temperature sensor in the battery pack or employing a "negative delta V" cutoff system. The temperature technique relies on detecting a sudden rise in battery temperature to shut off the charge. There is nothing inherently wrong with this approach, but battery packs do not always come with temperature sensors built in. Furthermore, those that do often sense the temperature of only one cell.
The negative delta V system utilizes the electrical characteristic that the NiCd/NiMH battery voltage peaks and drops about 20mV per cell when fully charged. This charger, in its basic configuration, will detect a peak of 84mV (per battery) from 2V to 21.5V, thus capable of charging any battery pack within this range (i.e., 6-12 cells or 7.2V to 14.4V). Consequently, no matter how discharged the battery is, this technique will provide sufficient charge to restore the battery to its full state, followed by a continuous "topping up" with a trickle charge to mitigate slow leakage through internal resistance.
Additional considerations include the necessity of allowing a battery to cool down before applying a charge for optimal performance. The charger is designed to wait for the battery voltage to stabilize for approximately 30 seconds before initiating charging. If the battery has just come off discharge and is hot, it may take a minute or so for the charge to commence. Additionally, new batteries may exhibit false peaks during the initial 4 minutes of charging; therefore, the charger begins with a slow "soft start" charge for 4 minutes to allow the battery to cool and surpass this transient period.
The charger employs a threshold of 2V (open circuit voltage) to recognize the connection of a battery. In practice, even a very old battery that has been shorted out for some time will recover above this value when unloaded. The charging algorithm utilized by the PIC is structured to ensure systematic operation. During normal operation, the charger is activated, and both LEDs will flash once. The charger will then enter mode 0 (standby) until a battery is connected. Upon connection, the charger progresses through mode 1 (cool), mode 2 (soft), mode 3 (fast), and mode 4 (trickle). The battery can remain in mode 4 (trickle) indefinitely or be removed at that point. When the battery is removed, the charger will revert to mode 0 (standby).Here we use the PIC16711. Rechargeable battery capacity is rated in mAH (milliampere-hours). The total capacity of a battery is defined as "C", that is it can supply C mA for 1 hour, or 2C for 30 minutes etc. Charge rates can vary from trickle charges to keep the battery 'topped up' of 3.3% of C to 5% of C, a slow current charge of 10% of C to 20% of C or a fast charge of 50% of C to 100% of C.
Slow charges are not meant to be continually applied, and since NiCd/NiMH batteries are about 66% efficient, they normally last about 8-15 hours. Fast charges such as 100% of C should be terminated after about 1.5 hours, providing the battery is flat to begin with.
Once a battery is fully charged, the battery produces gas creating a high internal pressure, and a sudden rise in temperature. The charge should be switched to a trickle charge at this point or the battery will begin to vent and release its electrolyte. My old battery was rated at C=1300mAH and my old charger was rated 400mA (30% of C) so the charger should have been switched off after about 4 hours, provided that they were almost flat to begin with.
However there is no way of knowing if C was actually 1300maH or if it had decreased a bit, and once the a battery starts to deteriorate, I suspect this becomes a vicious cycle and the battery deteriorates rapidly due to more and more overcharging. The manufacturer suggests these cells should be good for 500 to 1000 cycles if properly treated! The Memory Effect Myth Possibly the biggest myth that exists partcularly for NiCd cells is the "memory effect".
Almost every one quotes it as the reason that cells have to be completely flattened - otherwise they develop some sort of memory, and can only hold a partial charge from there on. Like all good stories, this one has a grain of truth in it! The myth originated from the early days of satellites when they were using solar cells to charge batteries and because of the orbiting of the craft around the earth, the batteries were subjected to precise charge/discharge cycles many hundreds of times.
The effect disappears when the battery cycle is suddenly varied, and it is extremely difficult to reproduce this effect even in a laboratory. So the "memory effect" is not a significant problem in home usage. What I can tell you is while it may be OK to discharge individual cells to 0V, it is certainly not recommended to discharge an entire battery of cells.
The reason is simple. When the battery is discharged below 0.8V per cell, one of the cells is inevitably weaker than the others, and goes to zero first. If the battery is further flattened this battery becomes charged in reverse, which again makes it still weaker.
This creates a more common but less commonly known effect called "voltage depression". Eventually the battery's performance drops off quite suddenly which ironically is the very thing that the user is trying to prevent. Most users know where the battery's "knee" occurs; it is when the original equipment first starts to show signs that the battery performance (and hence voltage) is suddenly dropping, and it is a good idea to place it straight on charge at this point.
Usually there is less than 5% of C remaining anyway. One other thing, batteries don't like getting too hot or cold; they do not take a full charge and they actually discharge (even under no load) much faster when over 40 degrees or below 0 degrees. They can build up internal heat when working and this can cause temperatures inside to increase also.
Particularly avoid leaving cordless tools inside a hot car for this reason. They also should be left to cool down for a while after discharge before placing them on charge. NiCd/NiMH batteries do self-discharge too, as a rule of thumb a battery will hold a full charge (with no load) for about a month or two, although when they get old or hot, they might only last a day. So what can you learn from this? You don't have to flatten your battery before you recharge it, Don't flatten your battery below 0.8V per cell, Don't overcharge your battery beyond 100% of C, and NiCd/NiMH don't like to get too hot, or too cold (0 to 40 degrees C is usually best) NiCd/NiMH Charging Common values for C for cordless tools and racing cars are in the range from 1000maH to 3000maH.
The first step is to determine what C is for your cells. Inspect the cells or contact the manufacturer to determine the cell part number. In drills, the battery packs can often be disassembled easily. The value for C often forms some of the part number and the part number can be searched for on the Internet. For my new battery the value for C was 1700maH. Note that the cell value for C is the same as the battery value for C. Manufacturers data gives that when designing a charger you should first consider how the cells are to be used.
For these applications the charge use is termed "cycle use" where the battery is repeatedly charged and discharged. In addition, usually the charge time required is as fast as possible, between 1 and 2 hours. My batteries were capable of taking a fast charge of 100% of C, which equates to 1.7A. Despite this I conservatively selected 1.25A as my charge current, because I wished to be able to charge 1300maH batteries also.
This value should be good for most readers, and it doesn't really matter if it is a bit less than 100% of C, because the charger will still detect a peak eventually anyway. However, some readers will want to adjust the maximum current, and this is described a bit later on. For "cycle use", there are two recommended methods of detecting charge termination, either using a temperature sensor in the battery pack or using a "negative delta V" cutoff system.
The temperature technique relies on detecting the sudden rise in battery temperature to shut off the charge. There is nothing wrong with doing this but battery packs do not always come with temperature sensors built in.
Furthermore ones that do, often sense the temperature of only one cell. The negative delta V system relies on the electrical characteristic that the NiCd/NiMH battery voltage peaks and drops about 20mV per cell when fully charged. This charger in its basic configuration will detect a peak of 84mV (per battery) from 2V to 21.5V, and thus will charge any battery pack in this range (i.e.
6-12 cells or 7.2V to 14.4V). Options to alter this range are described in Figure 10. Hence, no matter how discharged the battery is, this technique will give enough charge to restore the battery to its full state, and then the battery is continually "topped up" with a trickle charge to prevent slow leakage through internal resistance. Other things to consider are the requirement to let a battery cool down, so a better charge can be applied.
This battery charger waits for the battery voltage to stabilize for about 30 seconds before starting to charge. If the battery has just come off discharge and is hot, it may take a minute or so for the charge to begin to start.
Additionally new batteries may show false peaks in the first 4 minutes of charge. For this reason the charger starts with a slow "soft start" charge for 4 minutes to allow the battery to cool and get past this point. The charger uses a threshold of 2V (open circuit voltage) to recognize that a battery has been connected.
In practice even a very old battery that has been actually shorted out for some time, will recover above this value when unloaded. The charging algorithm used by the PIC is shown in Fig 1. Note that the first Light Emitting Diode (LED) comes on continually whilst battery is undergoing the bulk charge process, while the second LED gives an indication of the particular type of charge being applied.
Normal operation of the charger is fairly straightforward. Normally the Charger is switched on and both LEDs will flash once. The charger will then wait in mode 0 (standby) until a battery is connected. Once a battery is connected, the charger will progress though mode 1 (cool), 2 (soft), 3 (fast) and 4 (trickle). The battery can be left in mode 4 (trickle) indefinitely, or removed at this point. When the battery is removed, the charger will revert to mode 0 (standby). 🔗 External reference