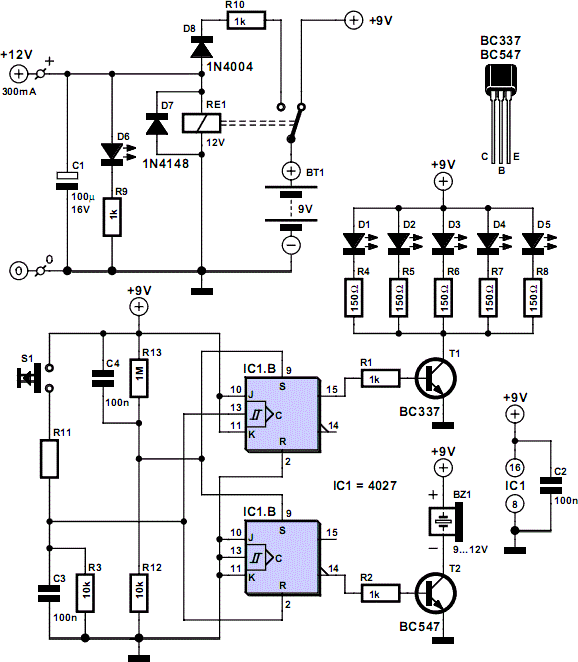
12 Volt fluorescent lamp drivers
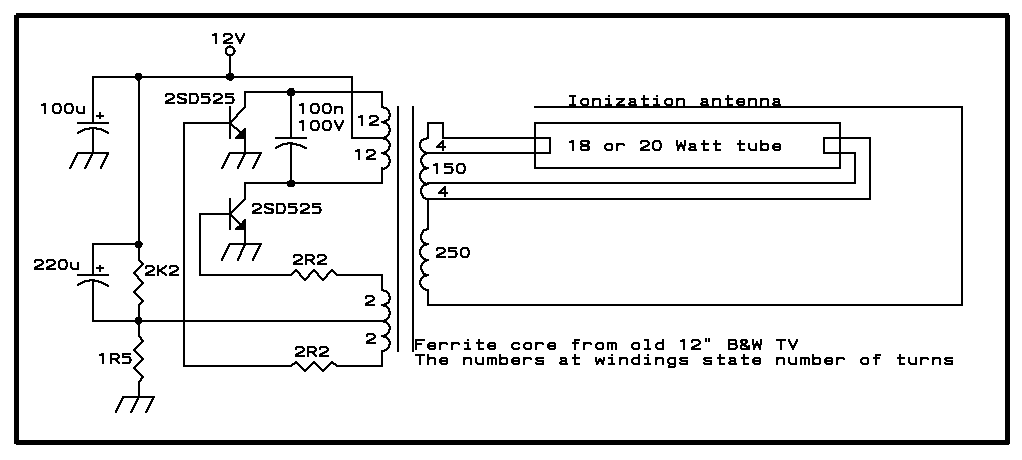
Whenever there is a need for battery-powered lighting, such as for camping, solar-powered cottages, cars, boats, planes, or emergency situations, fluorescent lamps are highly appealing. They are significantly more efficient than incandescent lamps, producing much more light for less power consumption. Additionally, the light color remains consistent as the battery depletes, unlike car lamps designed for 13.8 volts, which emit a yellow-orange hue under typical battery conditions. This is due to voltage drops that can reduce the voltage at the lamp to around 11 volts. Consequently, many manufacturers have developed fluorescent lamp drivers for battery use, accommodating input voltages of 6, 12, or 24 volts. There are also complete fluorescent lights available that operate on flashlight batteries; however, many of these low-cost options do not drive the fluorescent tubes effectively, resulting in low light output and efficiency comparable to incandescent lamps. This issue arises from consumer preferences for cheaper products rather than considering specifications and investing in higher-quality options. This article provides insights and presents three drivers for 12 volts: one for 20 watts, one for 8 watts, and one that delivers 2 watts to a 4-watt tube. A typical fluorescent lamp consists of a glass tube filled with low-pressure mercury vapor and other gases, with filaments at each end. The tube is coated internally with fluorescent salts. During operation, an arc forms within the tube, causing the mercury to emit ultraviolet light, which excites the fluorescent materials to produce visible light. The color of the emitted light depends on the specific mix of fluorescent materials used.
From an electrical perspective, driving such a lamp poses challenges. When off, the gas does not conduct electricity, and the cold filaments exhibit very low resistance. Initially, a strong current (typically 2 to 4 volts) is applied to the filaments to heat them. Once heated, a high-voltage pulse is required to ionize the gas, with necessary voltages ranging from approximately 100 to 10,000 volts, depending on various factors such as length, type, age, and temperature of the lamp. Once ionization occurs, the voltage drops significantly, and the tube requires a controlled current of approximately 0.1 to 0.3 amperes, averaging around 100 volts per meter of tube length. Maintaining this current is complex due to the negative resistance characteristic of the tube, where increasing current decreases voltage drop. Additionally, alternating current (AC) is essential to prevent one filament from cooling while the other overheats, which can lead to filament evaporation and darkening at one end of the tube.
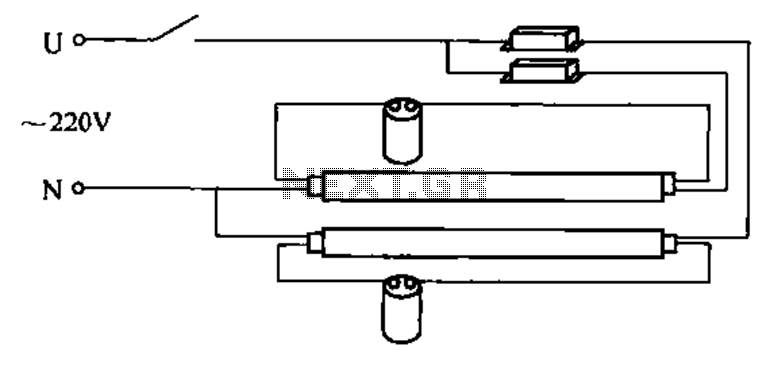
Historically, to meet these requirements for office lighting, an electrical engineer developed a straightforward solution: a series circuit comprising the AC power line, a ballast inductor, one filament, a starter device, the other filament, and a return path to the AC line. Initially, the starter maintains a closed contact, allowing the ballast to limit current flow through the filaments. As the filaments heat up, a bimetal within the starter also heats up. Eventually, the starter opens its contact. If this occurs while the current through the ballast is near its peak, a high-voltage pulse is generated by the collapse of the magnetic field, igniting the tube. Conversely, if it happens near a current minimum, the tube may not fully ionize, leading to current cessation, cooling of the filaments and starter, and the starter closing again, restarting the cycle. This mechanism causes the familiar flickering observed when turning on such lights. Once the tube ignites, the ballast regulates the current flowing through the tube, ensuring stable operation.Whenever there is a need for battery-powered lighting, like for camping, solar powered cottages, cars, boats, planes, or emergency purposes, fluorescent lamps have a great appeal. Firstly, they are very much more efficient than glow lamps, so they produce much more light for less power consumption.
Secondly, their light color stays constant while the battery runs down, while the color produced by a car lamp, really designed for 13. 8 Volt, is very yellow-orange under the typical battery-light conditions: 12 V at the battery, minus the voltage drop on some meters of cable, usually results in only 11 V or so at the lamp. This has lead to many manufacturers producing fluorescent lamp drivers for battery use, generally accepting input voltages of 6, 12, or 24 Volt.
Also there are a lot of complete fluorescent lights available, powered by flashlight batteries. But most of these cheap things have a quirk: They don`t drive the fluorescent tubes correctly, resulting in low light output, and an efficiency that is not so much better than that of a glow lamp. The manufacturers are not to be blamed for this: The culprit is the public, which prefers buying the cheapest thing available, instead of checking for specifications and paying 20% more for something that is 200% better.
In this article I will offer a little insight, and offer three drivers for 12 V that I`m using myself: One for 20 Watt, one for 8 Watt, and one that puts 2 Watt into a 4 Watt tube. A typical fluorescent lamp is basically a glass tube, filled with low pressure mercury vapor and a mix of additional gases, and having a filament at each end.
The glass tube is internally coated with fluorescent salts. In use, an arc forms in the tube, the mercury emits ultraviolet light, this excites the fluorescent substances, which in turn produce visible light. The exact color of the light depends on the mix of different fluorescent substances used. From an electrical point of view, such a lamp is a terrible thing to drive. While it is off, the gas does not conduct, and the cold filaments have a very low resistance. The first thing when powering up a tube is putting a rather strong current into these filaments, at very low voltage (2 to 4 V is typical).
When the filaments have heated up, the tube needs a high voltage pulse to ionize the gas. Depending on the length, type, age and temperature, the voltage required can be anything from about 100 to 10000 V. Once the gas ionizes, the voltage breaks down dramatically. From then on, the tube needs a controlled current of typically 0. 1 to 0. 3 A, depending on its diameter, at a voltage that depends on length and other factors, and averages to about 100 V per meter of tube length.
The current must be reasonably controlled, which isn`t easy, because the tube exhibits negative resistance: Increasing the current decreases the voltage drop! And it must be AC, because otherwise one filament cools off while the other overheats, evaporates and darkens one end of the tube.
For office lighting, many years ago some electrical engineer found a simple way to satisfy these needs: A series circuit is made from the AC power line through a ballast inductor, one filament, a starter device, the other filament, and back to the AC line. At first the starter has a closed contact, and the ballast limits the current that flows through the filaments.
They heat up, and so does a bimetal in the starter. Suddenly the starter opens. If this happens while the current in the ballast was near a peak, a high voltage pulse is produced by the magnetic field collapse, and the tube ignites. If it happens near a current minimum, the tube doesn`t ionize fully, the current ceases, the filaments and starter cool off, the starter closes and the cycle starts anew.
This leads to the well known flickering when switching on such a light. Once the tube has ignited, the ballast limits the current flowing through the tube, t 🔗 External reference
From an electrical perspective, driving such a lamp poses challenges. When off, the gas does not conduct electricity, and the cold filaments exhibit very low resistance. Initially, a strong current (typically 2 to 4 volts) is applied to the filaments to heat them. Once heated, a high-voltage pulse is required to ionize the gas, with necessary voltages ranging from approximately 100 to 10,000 volts, depending on various factors such as length, type, age, and temperature of the lamp. Once ionization occurs, the voltage drops significantly, and the tube requires a controlled current of approximately 0.1 to 0.3 amperes, averaging around 100 volts per meter of tube length. Maintaining this current is complex due to the negative resistance characteristic of the tube, where increasing current decreases voltage drop. Additionally, alternating current (AC) is essential to prevent one filament from cooling while the other overheats, which can lead to filament evaporation and darkening at one end of the tube.
Historically, to meet these requirements for office lighting, an electrical engineer developed a straightforward solution: a series circuit comprising the AC power line, a ballast inductor, one filament, a starter device, the other filament, and a return path to the AC line. Initially, the starter maintains a closed contact, allowing the ballast to limit current flow through the filaments. As the filaments heat up, a bimetal within the starter also heats up. Eventually, the starter opens its contact. If this occurs while the current through the ballast is near its peak, a high-voltage pulse is generated by the collapse of the magnetic field, igniting the tube. Conversely, if it happens near a current minimum, the tube may not fully ionize, leading to current cessation, cooling of the filaments and starter, and the starter closing again, restarting the cycle. This mechanism causes the familiar flickering observed when turning on such lights. Once the tube ignites, the ballast regulates the current flowing through the tube, ensuring stable operation.Whenever there is a need for battery-powered lighting, like for camping, solar powered cottages, cars, boats, planes, or emergency purposes, fluorescent lamps have a great appeal. Firstly, they are very much more efficient than glow lamps, so they produce much more light for less power consumption.
Secondly, their light color stays constant while the battery runs down, while the color produced by a car lamp, really designed for 13. 8 Volt, is very yellow-orange under the typical battery-light conditions: 12 V at the battery, minus the voltage drop on some meters of cable, usually results in only 11 V or so at the lamp. This has lead to many manufacturers producing fluorescent lamp drivers for battery use, generally accepting input voltages of 6, 12, or 24 Volt.
Also there are a lot of complete fluorescent lights available, powered by flashlight batteries. But most of these cheap things have a quirk: They don`t drive the fluorescent tubes correctly, resulting in low light output, and an efficiency that is not so much better than that of a glow lamp. The manufacturers are not to be blamed for this: The culprit is the public, which prefers buying the cheapest thing available, instead of checking for specifications and paying 20% more for something that is 200% better.
In this article I will offer a little insight, and offer three drivers for 12 V that I`m using myself: One for 20 Watt, one for 8 Watt, and one that puts 2 Watt into a 4 Watt tube. A typical fluorescent lamp is basically a glass tube, filled with low pressure mercury vapor and a mix of additional gases, and having a filament at each end.
The glass tube is internally coated with fluorescent salts. In use, an arc forms in the tube, the mercury emits ultraviolet light, this excites the fluorescent substances, which in turn produce visible light. The exact color of the light depends on the mix of different fluorescent substances used. From an electrical point of view, such a lamp is a terrible thing to drive. While it is off, the gas does not conduct, and the cold filaments have a very low resistance. The first thing when powering up a tube is putting a rather strong current into these filaments, at very low voltage (2 to 4 V is typical).
When the filaments have heated up, the tube needs a high voltage pulse to ionize the gas. Depending on the length, type, age and temperature, the voltage required can be anything from about 100 to 10000 V. Once the gas ionizes, the voltage breaks down dramatically. From then on, the tube needs a controlled current of typically 0. 1 to 0. 3 A, depending on its diameter, at a voltage that depends on length and other factors, and averages to about 100 V per meter of tube length.
The current must be reasonably controlled, which isn`t easy, because the tube exhibits negative resistance: Increasing the current decreases the voltage drop! And it must be AC, because otherwise one filament cools off while the other overheats, evaporates and darkens one end of the tube.
For office lighting, many years ago some electrical engineer found a simple way to satisfy these needs: A series circuit is made from the AC power line through a ballast inductor, one filament, a starter device, the other filament, and back to the AC line. At first the starter has a closed contact, and the ballast limits the current that flows through the filaments.
They heat up, and so does a bimetal in the starter. Suddenly the starter opens. If this happens while the current in the ballast was near a peak, a high voltage pulse is produced by the magnetic field collapse, and the tube ignites. If it happens near a current minimum, the tube doesn`t ionize fully, the current ceases, the filaments and starter cool off, the starter closes and the cycle starts anew.
This leads to the well known flickering when switching on such a light. Once the tube has ignited, the ballast limits the current flowing through the tube, t 🔗 External reference
Warning: include(partials/cookie-banner.php): Failed to open stream: Permission denied in /var/www/html/nextgr/view-circuit.php on line 713
Warning: include(): Failed opening 'partials/cookie-banner.php' for inclusion (include_path='.:/usr/share/php') in /var/www/html/nextgr/view-circuit.php on line 713