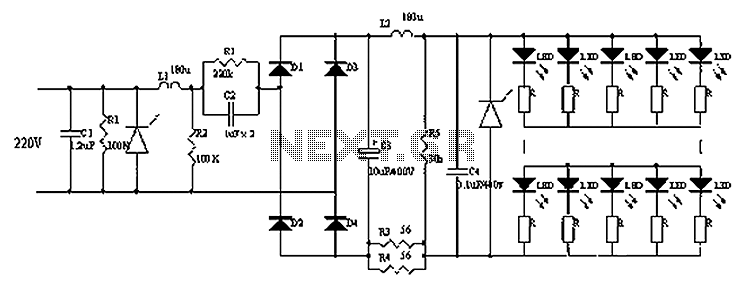
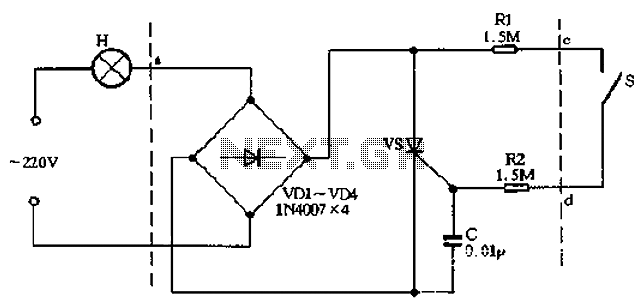
Fluorescent lamp
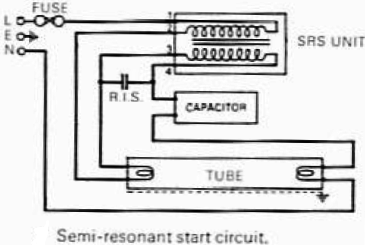
The F71T12 100 W bi-pin lamp is commonly used in tanning beds. It contains mercury, as indicated by the (Hg) symbol, which is now a requirement on all fluorescent lamps containing mercury in the United States. The lamp features a preheat design where the filament is encased in an oblong metal cathode shield to minimize end darkening. A fluorescent lamp, or fluorescent tube, operates as a low-pressure mercury-vapor gas-discharge lamp that utilizes fluorescence to emit visible light. When an electric current flows through the gas, it excites the mercury vapor, producing short-wave ultraviolet light that activates a phosphor coating inside the bulb, causing it to glow. Fluorescent lamps convert electrical energy into light much more efficiently than incandescent lamps, with a luminous efficacy that can exceed 100 lumens per watt, significantly higher than that of incandescent bulbs with similar light output. Although fluorescent fixtures are more expensive due to the need for a ballast to regulate current, the reduced energy costs typically compensate for the higher initial investment. Compact fluorescent lamps, available in sizes comparable to incandescent bulbs, serve as an energy-efficient alternative for residential use. Given their mercury content, many fluorescent lamps are classified as hazardous waste, leading the United States Environmental Protection Agency to recommend their separation from general waste for recycling or safe disposal. The phenomenon of fluorescence was observed in certain rocks and substances for centuries before its nature was understood. In the mid-19th century, it was noted that a radiant glow emerged from partially evacuated glass vessels when an electric current was applied. Sir George Stokes, an Irish scientist, was among the first to explain this phenomenon, coining the term "fluorescence" after fluorite, a mineral known for its glowing properties. This understanding was built upon the work of British scientists Michael Faraday and James Clerk Maxwell regarding electricity and light phenomena. The exploration of fluorescence advanced significantly in 1856 when Heinrich Geissler, a German glassblower, developed a mercury vacuum pump capable of creating a high vacuum in glass tubes. When an electrical current was passed through a Geissler tube, a strong green glow was observed at the cathode end, leading to its popularity for both entertainment and scientific inquiry. Julius Plücker was one of the first to systematically study the luminescent effects in Geissler tubes, noting the glow's shift in the presence of an electromagnetic field. Alexandre Edmond Becquerel discovered that certain materials emitted light when placed in a Geissler tube, prompting him to apply luminescent coatings to the tubes' surfaces. Although fluorescence was achieved, the initial tubes were inefficient and had short lifespans. Continued research into vacuum technologies led to the development of the Crookes tube, which was essential in the discovery of the electron by J. J. Thomson in 1897 and X-rays by Wilhelm Roentgen in 1895. However, the Crookes tube produced minimal light due to the highly effective vacuum created within.
The F71T12 100 W bi-pin lamp operates on the principles of gas discharge and fluorescence, making it an efficient light source for applications such as tanning beds. The bi-pin configuration allows for easy installation and secure electrical connections. The lamp's design includes a phosphor coating that is excited by ultraviolet light generated from the mercury vapor, resulting in visible light output. The use of a ballast is critical in fluorescent lamp systems, as it regulates the electrical current to prevent fluctuations that could lead to lamp failure or reduced lifespan.
In terms of safety and environmental considerations, the presence of mercury necessitates proper handling and disposal of fluorescent lamps to mitigate environmental hazards. The recommended recycling protocols help ensure that the hazardous materials are managed appropriately, aligning with regulations set forth by environmental agencies.
Overall, the F71T12 100 W bi-pin lamp exemplifies advancements in lighting technology, combining efficiency with specific applications while highlighting the importance of responsible usage and disposal practices in the context of environmental safety.Typical F71T12 100 W bi-pin lamp used in tanning beds. Note the (Hg) symbol indicating it contains mercury. In the US this symbol is now required on all fluorescent lamps that contain mercury. [1] Inside the lamp end of a preheat bi-pin lamp. In this lamp the filament is surrounded by an oblong metal cathode shield, which helps reduce lamp end da rkening. [2] A fluorescent lamp or fluorescent tube is a low pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor which produces short-wave ultraviolet light that then causes a phosphor coating on the inside of the bulb to glow.
A fluorescent lamp converts electrical energy into useful light much more efficiently than incandescent lamps. The luminous efficacy of a fluorescent light bulb can exceed 100 lumens per watt, several times the efficacy of an incandescent bulb with comparable light output.
Fluorescent lamp fixtures are more costly than incandescent lamps because they require a ballast to regulate the current through the lamp, but the lower energy cost typically offsets the higher initial cost. Compact fluorescent lamps are now available in the same popular sizes as incandescents and are used as an energy-saving alternative in homes.
Because they contain mercury, many fluorescent lamps are classified as hazardous waste. The United States Environmental Protection Agency recommends that fluorescent lamps be segregated from general waste for recycling or safe disposal. [3] Fluorescence of certain rocks and other substances had been observed for hundreds of years before its nature was understood.
By the middle of the 19th century, experimenters had observed a radiant glow emanating from partially evacuated glass vessels through which an electric current passed. One of the first to explain it was the Irish scientist Sir George Stokes from the University of Cambridge, who named the phenomenon "fluorescence" after fluorite, a mineral many of whose samples glow strongly due to impurities.
The explanation relied on the nature of electricity and light phenomena as developed by the British scientists Michael Faraday in the 1840s and James Clerk Maxwell in the 1860s. [4] Little more was done with this phenomenon until 1856 when a German glassblower named Heinrich Geissler created a mercury vacuum pump that evacuated a glass tube to an extent not previously possible.
When an electrical current passed through a Geissler tube, a strong green glow on the walls of the tube at the cathode end could be observed. Because it produced some beautiful light effects, the Geissler tube was a popular source of amusement.
More important, however, was its contribution to scientific research. One of the first scientists to experiment with a Geissler tube was Julius PlG cker who systematically described in 1858 the luminescent effects that occurred in a Geissler tube. He also made the important observation that the glow in the tube shifted position when in proximity to an electromagnetic field.
Alexandre Edmond Becquerel observed in 1859 that certain substances gave off light when they were placed in a Geissler tube. He went on to apply thin coatings of luminescent materials to the surfaces of these tubes. Fluorescence occurred, but the tubes were very inefficient and had a short operating life. Inquiries that began with the Geissler tube continued as even better vacuums were produced. The most famous was the evacuated tube used for scientific research by William Crookes. That tube was evacuated by the highly effective mercury vacuum pump created by Hermann Sprengel. Research conducted by Crookes and others ultimately led to the discovery of the electron in 1897 by J.
J. Thomson and X-rays in 1895 by Wilhelm Roentgen. But the Crookes tube, as it came to be known, produced little light because the vacuum in it was too good and thus lacked the trace amounts o 🔗 External reference
The F71T12 100 W bi-pin lamp operates on the principles of gas discharge and fluorescence, making it an efficient light source for applications such as tanning beds. The bi-pin configuration allows for easy installation and secure electrical connections. The lamp's design includes a phosphor coating that is excited by ultraviolet light generated from the mercury vapor, resulting in visible light output. The use of a ballast is critical in fluorescent lamp systems, as it regulates the electrical current to prevent fluctuations that could lead to lamp failure or reduced lifespan.
In terms of safety and environmental considerations, the presence of mercury necessitates proper handling and disposal of fluorescent lamps to mitigate environmental hazards. The recommended recycling protocols help ensure that the hazardous materials are managed appropriately, aligning with regulations set forth by environmental agencies.
Overall, the F71T12 100 W bi-pin lamp exemplifies advancements in lighting technology, combining efficiency with specific applications while highlighting the importance of responsible usage and disposal practices in the context of environmental safety.Typical F71T12 100 W bi-pin lamp used in tanning beds. Note the (Hg) symbol indicating it contains mercury. In the US this symbol is now required on all fluorescent lamps that contain mercury. [1] Inside the lamp end of a preheat bi-pin lamp. In this lamp the filament is surrounded by an oblong metal cathode shield, which helps reduce lamp end da rkening. [2] A fluorescent lamp or fluorescent tube is a low pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor which produces short-wave ultraviolet light that then causes a phosphor coating on the inside of the bulb to glow.
A fluorescent lamp converts electrical energy into useful light much more efficiently than incandescent lamps. The luminous efficacy of a fluorescent light bulb can exceed 100 lumens per watt, several times the efficacy of an incandescent bulb with comparable light output.
Fluorescent lamp fixtures are more costly than incandescent lamps because they require a ballast to regulate the current through the lamp, but the lower energy cost typically offsets the higher initial cost. Compact fluorescent lamps are now available in the same popular sizes as incandescents and are used as an energy-saving alternative in homes.
Because they contain mercury, many fluorescent lamps are classified as hazardous waste. The United States Environmental Protection Agency recommends that fluorescent lamps be segregated from general waste for recycling or safe disposal. [3] Fluorescence of certain rocks and other substances had been observed for hundreds of years before its nature was understood.
By the middle of the 19th century, experimenters had observed a radiant glow emanating from partially evacuated glass vessels through which an electric current passed. One of the first to explain it was the Irish scientist Sir George Stokes from the University of Cambridge, who named the phenomenon "fluorescence" after fluorite, a mineral many of whose samples glow strongly due to impurities.
The explanation relied on the nature of electricity and light phenomena as developed by the British scientists Michael Faraday in the 1840s and James Clerk Maxwell in the 1860s. [4] Little more was done with this phenomenon until 1856 when a German glassblower named Heinrich Geissler created a mercury vacuum pump that evacuated a glass tube to an extent not previously possible.
When an electrical current passed through a Geissler tube, a strong green glow on the walls of the tube at the cathode end could be observed. Because it produced some beautiful light effects, the Geissler tube was a popular source of amusement.
More important, however, was its contribution to scientific research. One of the first scientists to experiment with a Geissler tube was Julius PlG cker who systematically described in 1858 the luminescent effects that occurred in a Geissler tube. He also made the important observation that the glow in the tube shifted position when in proximity to an electromagnetic field.
Alexandre Edmond Becquerel observed in 1859 that certain substances gave off light when they were placed in a Geissler tube. He went on to apply thin coatings of luminescent materials to the surfaces of these tubes. Fluorescence occurred, but the tubes were very inefficient and had a short operating life. Inquiries that began with the Geissler tube continued as even better vacuums were produced. The most famous was the evacuated tube used for scientific research by William Crookes. That tube was evacuated by the highly effective mercury vacuum pump created by Hermann Sprengel. Research conducted by Crookes and others ultimately led to the discovery of the electron in 1897 by J.
J. Thomson and X-rays in 1895 by Wilhelm Roentgen. But the Crookes tube, as it came to be known, produced little light because the vacuum in it was too good and thus lacked the trace amounts o 🔗 External reference